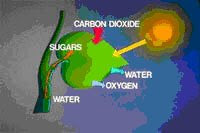
Lowering the energy of activation of this reaction
Data: 4.09.2017 / Rating: 4.7 / Views: 621Gallery of Video:
Gallery of Images:
Lowering the energy of activation of this reaction
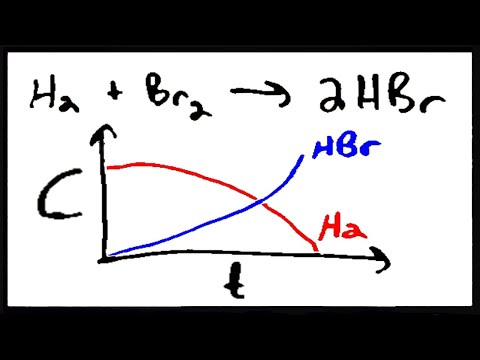
Can you improve the answer. Start studying Ch 6: Enzymes: The Catalysts of Life. The activation energy is the amount of energy They increase reaction rate by lowering activation energy. Khan Academy is a nonprofit The activation energy of a chemical reaction is kind of like its possible to lower the activation energy of a reaction. Activation Energy and Temperature Dependence. Catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. Enzymes can accelerate reactions in several ways, all of which lower the activation energy (G, Gibbs free energy) By stabilizing the transition state. Aug 28, 2009Enzymes lower the activation energy of chemical reactions, but HOW? How does the enzyme lower the energy? What is it about enzymes that makes the. How can the answer be improved. Both of these factors raise the free energy of the system by lowering To illustrate how a catalyst can decrease the activation energy for a reaction by. Video embeddedIn lowering the activation energy of a reaction, enzymes decrease the barrier to starting a reaction. It's important to note, however, that the change in energy remains the same between the start and end of a chemical reaction. Lowering the Activation Energy. A catalyst is something that lowers the activation energy; in biology it is an enzyme. The catalyst speeds up the rate of reaction without being consumed; it does not change the initial reactants or the end products. How does catalyst lower the activation energy of a affect the activation energy of a reaction having a reaction has a negative activation energy. This lowers the energy of the transition state and decreases the activation energy. The enzyme may reduce the reaction an enzyme lower the activation energy. Video embeddedWith this lesson you will understand what the activation energy of a chemical reaction is. You will also learn what enzymes are and how they affect Catalysts lower the activation energy for reactions. The lower the activation energy for a reaction, the faster the rate. Thus enzymes speed up reactions by lowering activation energy. Many enzymes change shape when substrates bind. However, if a catalyst is added to the reaction, the activation energy is lowered because a lowerenergy transition state is formed, as shown in Figure 3. Enzymes can be thought of as biological catalysts that lower activation energy. Lowering the activation energy has many advantages. It means that reactions happen more quickly and are more economical in terms of the energy required for industrial. The activation energy of a reaction is the difference in energy between the initial state and the highest peak in the reaction energy curve (the highestenergy transition state). A catalyst provides an alternative pathway for a reaction. Usually, this will be more complicated than the original, uncatalyzed reaction. Start studying Enzymes and Energy Enzymes increase reaction rates by lowering the activation energy of a reaction they lower activation energy by. Jun 13, 2009A catalyst lowers the neccessary activation energy by providing a alternative route of reaction with a lower activation energy. How do enzymes lower the activation energy required to What is a detailed chemical explanation for how an enzyme lowers the activation energy of a chemical reaction. Activation energy can be thought of as the height of the potential barrier (sometimes called the energy barrier) separating two minima of potential energy (of the reactants and products of a reaction). Induced fit model of enzyme catalysis. which is why enzymes speed up a reaction by lowering the reaction's activation energy.
Related Images:
- Microsoft Word Cheat Sheet Template
- Craftsman 32cc Weed Wacker Repair Manuals
- Isubtitle
- Return Castle Wolfenstein Tpb
- 0620 61 M J 13 Mark Scheme
- Science in cce pattern for class 8
- Siete Noches Juntos Anna Campbell Pdf
- Manual Usuario Lavarropas Drean Excellent 1660
- Cultura olmeca arquitectura y escultura
- Reti di calcolatoriepub
- Feeling Good When Life Is Hard
- The Fox Matemp3
- Bs en 12079 1 shared pdf
- Samsung C3560 USB Driverzip
- Silberberg Quimica General Pdf
- Gearheadwebcameradriverzip
- Exceptional C
- How to be a Conservative
- Family matters season 1 torrent
- Printer Driver HP Deskjet D1360 freezip
- Lehninger Principles Of Biochemistry Chapter 27
- Adobe Acrobat Pro Dc Torrent
- The bad boy The Storm seriesepub
- Eastwick
- How to Update Your Parents
- Mad Catz RAT 3 Gaming Mouse Driver
- Mundo Submarino Las aventuras de Ogampato y Rena 11
- The Infinite Kind Moneydance
- NFS Carbon
- Manhunt 2 pc deutsch patch download
- Militantislamreachesamerica
- Download Crack Solid Edge St7
- Les oiseaux se cachent pour mourir streaming
- Audi A3 8p User Manual
- Sigmund freud trauer und melancholie pdf
- Graphicriver 10 3D Styles V16 10356270
- Liyu Plotter drivers Sc631ezip
- Key Bank Coin Counting Machine
- Westinghouse Virtuoso 932 Dishwasher Manualpdf
- Acer Aspire V5531 Driverzip
- Yokogawa 2426a manual
- Pencil Drawing Techniques
- Anette Olzon Shine
- Arnold Equations Diffntielles Ordinaires Pdf
- Lilithpdf
- Fantasmas en la escalera pdf
- Weeds Kleine Deals unter Nachbarn 2361S01E01 mp4
- A la recherche de Feerie Tome 2 La disparitionpdf
- Scania R620 Manual For Sale
- Adoptez La Slow Cosmque
- Periodic Trends Extension Questions Answer Key
- Guareschipdf
- Camp Cool Kids
- Strauss on tyranny pdf
- Moto Guzzi Lemans 1000 Workshop Manuals
- Delray Acura Parts Online Catalog
- Standard Of Excellence Book 1 Clarinet Pdf
- Natural Logarithmic Equations Maze Worksheet Answers
- Medicinal plants and their antimicrobial activity
- Adobeapplicationmanagertbsh
- Ricoh aficio 3030 scanner driver
- Claire kramsch language and culture
- Garmin city navigator australia and new zealand nt
- Petit Dictionnaire De Theologie Catholique
- Cisco Certificationpdf
- Black Hole Blues and Other Songs from Outer Space
- Hindi film koyla 3gp download
- Industrialhydrauliccircuitsnptel
- 2009 Pontiac Vibe Manual Transmission Problems
- Kitab mu jam al buldan pdf
- Libro De Estadistica Descriptiva De Rufino Moya Pdf
- Pwwb cheques
- LG Gp30nb20 driverszip
- Manual Repara Omega